A total of 57 samples were taken for this research. In each sampling location, electrical conductivity (EC) and pH values were measured. The water samples were analyzed at the Center of Water and Environmental Sanitation (Centro de Aguas y Saneamiento Ambiental) from the University Mayor de San Simon in Bolivia. The parameters analyzed include major ions: calcium (Ca2+), magnesium (Mg2+), sodium (Na+), bicarbonate (HCO3–), chloride (Cl–), sulfate (SO42-); and minor ions such as potassium (K+), nitrate (NO3–), total iron (Fetotal), fluoride (F–), silicon dioxide (SiO2) and phosphate (PO43), the last three ions were collected only for 25 samples. The concentration of all parameters is in milligram per liter (mg/L), with exception of electrical conductivity (EC) which is in micro siemens per centimeter (µS/cm). The water sampling followed the protocol and standards methods of APHA (1995). The stable isotopes (2H and 18O) analyses were carried out at the laboratory of the Geological Survey of Denmark and Greenland. The isotopic compositions are reported as the deviation (‘δ’) of the ratio of the heavy isotopes (18O and 2H) to the light ones (16O and 1H) of a sample from that of the VSMOW (Vienna Standard Mean Ocean Water) in parts per thousand ‰.
The major ions analysis showed an increase in the Total Dissolved Solids (TDS) towards the middle and distal part of the fam, which is in agreement with the groundwater flow direction. Some of the samples have high concentrations of Na+ and Cl–, suggesting that there is a saline water layer within the aquifer system.
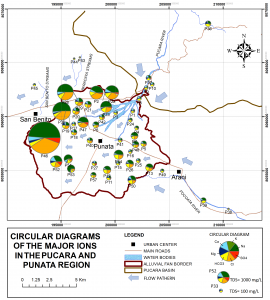
Several hypotheses were analyzed in order to identify the origin of saline water within the aquifer system. For instance, the hypothesis of halite dissolution was rejected due to an incompatible behavior on the Na+ and Cl– concentration and strengthen by the negative saturation indexes. In the other hand, the ions concentration among the samples suggested that there might be a ion exchange within freshwater and an exchangeable layer.
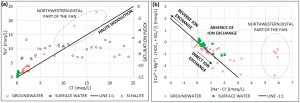
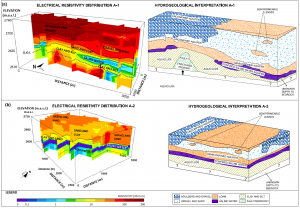
The ERT surveys showed a bottom layer composed of clay, which is an ideal exchangeable ion site. Moreover, the TEM surveys confirmed the presence of a very low resistivity layer, which is associate to saline water which is the source of the high concentrations of Na+ and Cl–.
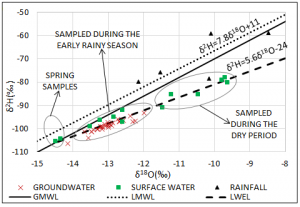
The isotopic analysis was useful for identifying the main groundwater recharge mechanism in the Punata alluvial fan. Surface samples are scattered in two main groups, maybe due to the fact that they were sampled in two different seasons (i.e. dry and rainy season). The groundwater samples are scattered mainly in one group and have similar composition than surface water samples during the rainy season. Hence it is very likely that groundwater is recharge by flash floods from the Pucara and Pocoata rivers.
For more detailed information refer to:
